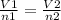Chemistry, 28.01.2020 09:31 granthazenp5e9mj
Gas laws describe and predict the behavior of gases without attempting to explain why they happen
true
false
a 6.0 l sample of nitrogen gas contains 0.50 mole of a gas. if enough gas is added to make a total of 0.75 moles at the same pressure and temperature, what is the resulting total volume of the gas? (4 points)
2.3 l
4.8 l
7.5 l
9.0 l

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, lizzyhearts
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3


Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Gas laws describe and predict the behavior of gases without attempting to explain why they happen
Questions in other subjects:



Mathematics, 06.03.2020 16:54