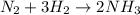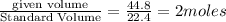Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

You know the right answer?
For the haber process, n2+3h2 yields 2nh3, what volume of nitrogen is consumed at stp if you collect...
Questions in other subjects:



Mathematics, 17.02.2021 01:10

Mathematics, 17.02.2021 01:10