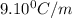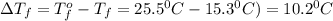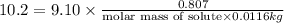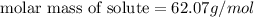Chemistry, 04.02.2020 20:46 Envious1552
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g of an unknown colorless liquid was dissolved in 11.6 g of tert-butyl alcohol, the solution froze at 15.3 ∘c. which of the following is most likely the identity of this unknown liquid? tert-butyl alcohol is a solvent with a of 9.10 and a freezing point of 25.5 . when 0.807 of an unknown colorless liquid was dissolved in 11.6 of tert-butyl alcohol, the solution froze at 15.3 .which of the following is most likely the identity of this unknown liquid? ethylene glycol (molar mass = 62.07 g/mol)1-octanol (molar mass = 130.22 g/mol)glycerol (molar mass = 92.09 g/mol)2-pentanone (molar mass = 86.13 g/mol)1-butanol (molar mass = 74.12 g/mol)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2
You know the right answer?
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g...
Questions in other subjects:










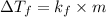
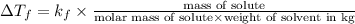
 = change in freezing point
= change in freezing point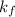 = freezing point constant=
= freezing point constant=