
Chemistry, 04.11.2019 11:31 christiantorres57
How will the osmotic pressure of an aqueous solution change as evaporation occurs?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2


Chemistry, 23.06.2019 17:30, dalejacksoniip5yf4y
Hydrogen-2 is also known as deuterium as well as hydrogen-3 is known as tritium hydrogen-1 is our common hydrogen isotope a sample hydrogen gas has 99% hydrogen -1 ,0.8% deuterium , and 0.2% tritium what is the average atomic mass of this mixture of isotope to the thousands place
Answers: 1
You know the right answer?
How will the osmotic pressure of an aqueous solution change as evaporation occurs?...
Questions in other subjects:

Mathematics, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

History, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01

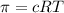
 = osmotic pressure
= osmotic pressure

