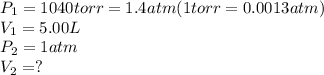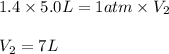Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
A5.00 l sample of a gas exerts a pressure of 1040 torr at 50.0°c. in what volume would the same samp...
Questions in other subjects:

Engineering, 16.10.2021 02:50


Mathematics, 16.10.2021 02:50

Health, 16.10.2021 02:50



Mathematics, 16.10.2021 02:50



Chemistry, 16.10.2021 02:50

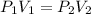
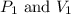 are initial pressure and volume.
are initial pressure and volume.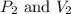 are final pressure and volume.
are final pressure and volume.