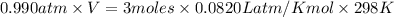Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, 4tazaouiamine1r
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Mg + hcl → mgcl2 + h2 how many liters of hydrogen gas is produced at 298 k and 0.990 atm if 6.00 mol...
Questions in other subjects:


Mathematics, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10

English, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10


Mathematics, 15.06.2021 18:10




 moles of hydrogen gas that is 3 moles.
moles of hydrogen gas that is 3 moles.