
Chemistry, 29.01.2020 17:45 firdausmohammed80
Reviewing for a test - need an answer and explanation.
base your answer to the following question on the information below.
the reaction between aluminum and an aqueous solution of gold (i) sulfate is represented by the unbalanced equation below.
al (s) + au2so4 (aq) -> al2(so4)3 (aq) + au (s)
determine the total mass of au sproduced when 6.52 grams of al reacts completely with 3.58 grams of au2so4 to produce 5.95 grams of al2(so4)3.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
You know the right answer?
Reviewing for a test - need an answer and explanation.
base your answer to the following...
base your answer to the following...
Questions in other subjects:



Mathematics, 03.12.2020 21:20


Biology, 03.12.2020 21:20



Mathematics, 03.12.2020 21:20

Business, 03.12.2020 21:20

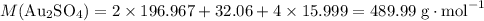 .
.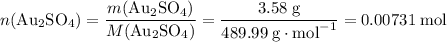 .
.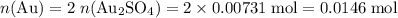 .
.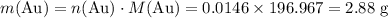 .
.

