

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
Consider the reaction below. at 500 k, the reaction is at equilibrium with the following concentrati...
Questions in other subjects:




Chemistry, 10.12.2019 04:31




Social Studies, 10.12.2019 04:31


Chemistry, 10.12.2019 04:31

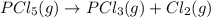
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0439/2265/73fe0.png)
![[PCl_5]](/tpl/images/0439/2265/29487.png) = 0.0095 M
= 0.0095 M ![[PCl_3]](/tpl/images/0439/2265/5e6c7.png) = 0.020 M
= 0.020 M![[Cl_2]](/tpl/images/0439/2265/53f7d.png) = 0.020 M
= 0.020 M ![K_c=\frac{[0.020][0.020]}{[0.0095]}=0.042](/tpl/images/0439/2265/3712e.png)


