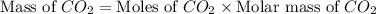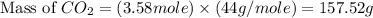Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
How many grams of co2 would be formed from 53.8 g of c2h6 and unlimited o2 in the reaction 2c2h6+7o2...
Questions in other subjects:

Social Studies, 22.07.2019 20:00

Mathematics, 22.07.2019 20:00

Biology, 22.07.2019 20:00


History, 22.07.2019 20:00

Mathematics, 22.07.2019 20:00

Social Studies, 22.07.2019 20:00



History, 22.07.2019 20:00

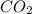 formed will be, 157.52 grams.
formed will be, 157.52 grams.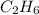 = 53.8 g
= 53.8 g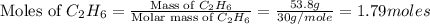
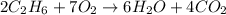
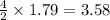 moles of
moles of