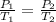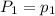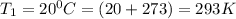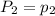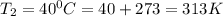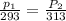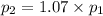Chemistry, 05.02.2020 02:52 alexandroperez13
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolute temperature t, volume v, and pressure p of a gas sample are related by the ideal gas law, which states that pv=nrt. here n is the number of moles in the gas sample and r is a gas constant that applies to all gases. this empirical law describes gases well only if they are sufficiently dilute and at a sufficiently high temperature that they are not on the verge of condensing. in applying the ideal gas law, p must be the absolute pressure, measured with respect to vacuum and not with respect to atmospheric pressure, and t must be the absolute temperature, measured in kelvins (that is, with respect to absolute zero, defined throughout this tutorial as ^ -273˚c). if p is in pascals and v is in cubic meters, use r=8.3145j/(mol x k). if p is in atmospheres and v is in liters, use r=0.08206l x atm/(mol x k) instead. part a a gas sample enclosed in a rigid metal container at room temperature (20.0˚c) has an absolute pressure p1. the container is immersed in hot water until it warms to 40.0˚c. what is the new absolute pressure p2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 01:30, josephaciaful
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolut...
Questions in other subjects:

Physics, 08.11.2019 03:31

Mathematics, 08.11.2019 03:31


Computers and Technology, 08.11.2019 03:31

Social Studies, 08.11.2019 03:31

Mathematics, 08.11.2019 03:31

History, 08.11.2019 03:31

Physics, 08.11.2019 03:31

Mathematics, 08.11.2019 03:31

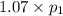
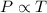 (At constant volume and number of moles)
(At constant volume and number of moles)