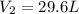Chemistry, 17.10.2019 15:20 naiquawhite
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, an...
Questions in other subjects:


English, 09.10.2019 15:00




Mathematics, 09.10.2019 15:00


Mathematics, 09.10.2019 15:00

Mathematics, 09.10.2019 15:00

History, 09.10.2019 15:00

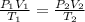
 = initial pressure of gas = 12 atm
= initial pressure of gas = 12 atm = final pressure of gas = 14 atm
= final pressure of gas = 14 atm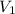 = initial volume of gas = 23 L
= initial volume of gas = 23 L = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas = 200K
= initial temperature of gas = 200K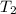 = final temperature of gas = 300K
= final temperature of gas = 300K