
Hello, everyone!
here is the problem i am stuck with.:
based on the “national ambient air quality objectives”, the acceptable hourly average concentration of carbon monoxide (co) is 35 mg/m^3.
find the acceptable concentration of co in ppm if the temperature is -30 °c and pressure is 0.92 atm. express the concentration as a percent by volume.
could someone me understand how to do it?
any input would be greatly appreciated!

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Hello, everyone!
here is the problem i am stuck with.:
based on the “national am...
here is the problem i am stuck with.:
based on the “national am...
Questions in other subjects:

Spanish, 10.12.2020 02:50


History, 10.12.2020 02:50


Mathematics, 10.12.2020 02:50


Physics, 10.12.2020 02:50



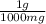 x
x 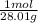
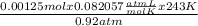
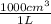 = 27.09 cm³
= 27.09 cm³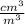 x
x 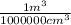 x 100%
x 100%

