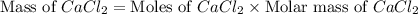Chemistry, 09.01.2020 00:31 nehakarakkattu
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure water at this temperature is 92.6 mmhg. what is the concentration of cacl2 in mass percent? "

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure wat...
Questions in other subjects:

Chemistry, 11.12.2020 03:20

Mathematics, 11.12.2020 03:20



Mathematics, 11.12.2020 03:20

Mathematics, 11.12.2020 03:20

Mathematics, 11.12.2020 03:20

English, 11.12.2020 03:20


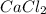 in mass percent is, 41.18 %
in mass percent is, 41.18 %
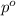 = vapor pressure of the pure component (water) = 92.6 mmHg
= vapor pressure of the pure component (water) = 92.6 mmHg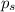 = vapor pressure of the solution = 83.1 mmHg
= vapor pressure of the solution = 83.1 mmHg = mole fraction of solute,
= mole fraction of solute, 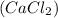

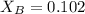
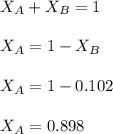
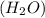 .
.