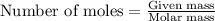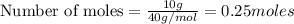Chemistry, 21.06.2019 19:00 cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1
You know the right answer?
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles,...
Questions in other subjects:

Mathematics, 05.12.2020 01:00

English, 05.12.2020 01:00

Social Studies, 05.12.2020 01:00


Geography, 05.12.2020 01:00



Biology, 05.12.2020 01:00

Physics, 05.12.2020 01:00