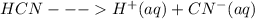Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

You know the right answer?
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is di...
Questions in other subjects:

Mathematics, 15.11.2019 06:31


Biology, 15.11.2019 06:31

Mathematics, 15.11.2019 06:31

Mathematics, 15.11.2019 06:31



Mathematics, 15.11.2019 06:31