Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1


Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
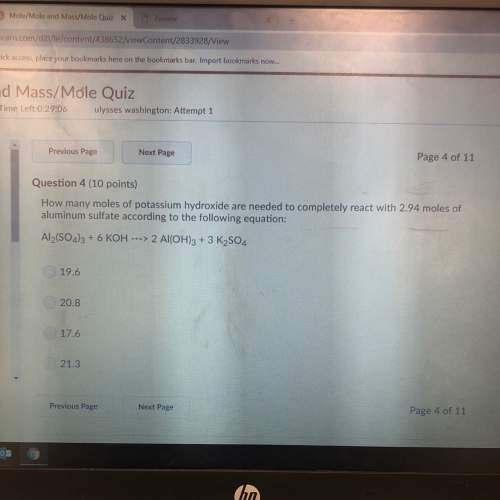
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sul...
Questions in other subjects:



History, 23.06.2019 18:30

English, 23.06.2019 18:30


Mathematics, 23.06.2019 18:30

English, 23.06.2019 18:30

Mathematics, 23.06.2019 18:30

Mathematics, 23.06.2019 18:30

Advanced Placement (AP), 23.06.2019 18:30