Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 19:30, jalenshayewilliams
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2
You know the right answer?
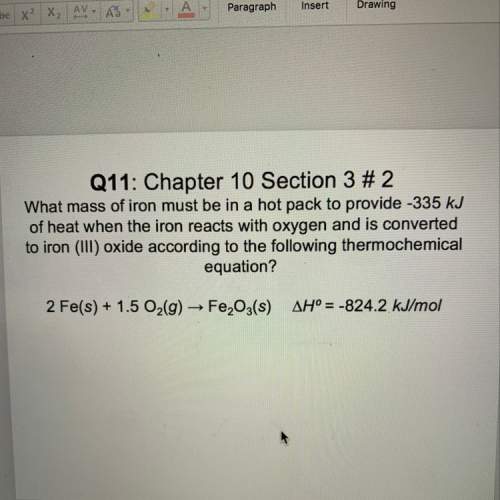
What mass of iron must be in a hot pack to provide -335kj of heat when the iron reacts with oxygen a...
Questions in other subjects:

Mathematics, 17.10.2019 11:30


Mathematics, 17.10.2019 11:30


Mathematics, 17.10.2019 11:30

English, 17.10.2019 11:30

Computers and Technology, 17.10.2019 11:30

History, 17.10.2019 11:30