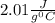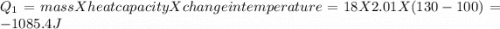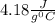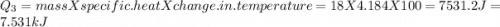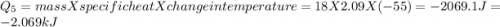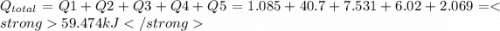Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 05:00, skylarjohnson2683
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 05:00, neidaq12345
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
How much heat (in kj) is evolved in converting 1.00 mol of steam at 130.0 ∘c to ice at -55.0 ∘c? th...
Questions in other subjects:










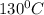 to
to 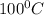 = Q1
= Q1![0^{0}C[/tex=Q3]4) conversion of water to ice=Q45) cooling of ice from [tex]0^{0}C](/tpl/images/0009/5828/c5d88.png) to
to 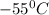 =Q5
=Q5