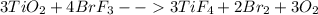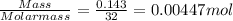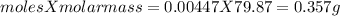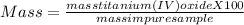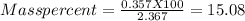Chemistry, 25.06.2019 02:00 Gearyjames8
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial chemical, is to combine the sample with bromine trifluoride to produce titanium ( iv) fluoride, liquid bromine , and oxygen has. suppose 2.367g of an impure sample( impure meaning that the sample has titanium (iv) oxide as well as other “ stuff” in it ) evolves 0.143 g of oxygen gas. what is the mass percent of titanium ( iv) oxide in the impure sample?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial che...
Questions in other subjects:

Mathematics, 01.03.2021 23:00


History, 01.03.2021 23:00

Computers and Technology, 01.03.2021 23:00





Mathematics, 01.03.2021 23:00