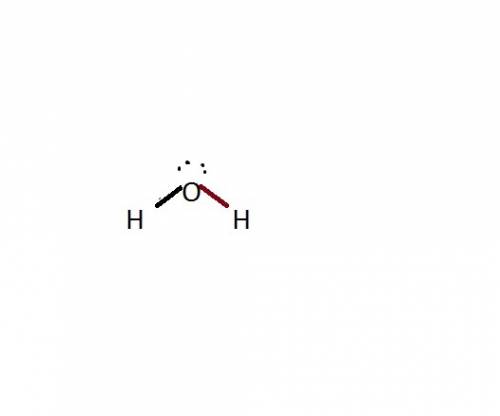
Chemistry, 25.06.2019 05:00 sarahaziz9526
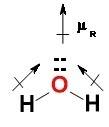
The common water molecule is polar because: it contains three polar covalent bonds. it contains one polar covalent bond. it contains no polar bonds, but is symmetrical. it has an asymmetrical shape.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

You know the right answer?
The common water molecule is polar because: it contains three polar covalent bonds. it contains one...
Questions in other subjects:





Mathematics, 04.12.2020 22:10

Law, 04.12.2020 22:10


Mathematics, 04.12.2020 22:10

Social Studies, 04.12.2020 22:10

![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0014/4487/08b6a.png)
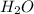
![{\text{Number of electrons}} =\frac{1}{2}[6+2-0+0]=4](/tpl/images/0014/4487/b0c43.png)
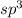 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.