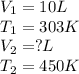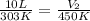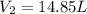Chemistry, 25.06.2019 11:00 Mintfu8122
Asample of krypton gas with a volume of 10.00 l has a temp of 303 k and exerts a pressure of 2.47 atm. what is the final volume if the temp increases to 450 k and pressure remains constant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
Asample of krypton gas with a volume of 10.00 l has a temp of 303 k and exerts a pressure of 2.47 at...
Questions in other subjects:

History, 19.05.2020 20:05

Mathematics, 19.05.2020 20:05




Mathematics, 19.05.2020 20:05




Chemistry, 19.05.2020 20:05

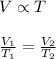
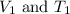 = Initial volume and temperature
= Initial volume and temperature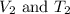 = Final volume and temperature
= Final volume and temperature