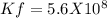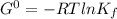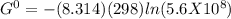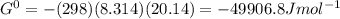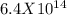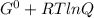Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which directio...
Questions in other subjects:

Mathematics, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

History, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

Mathematics, 15.09.2020 03:01

English, 15.09.2020 03:01

Physics, 15.09.2020 03:01