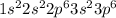Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3


Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1

Chemistry, 23.06.2019 11:30, kayabwaller4589
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1
You know the right answer?
The electron configuration of an element is shown below. 1s22s22p63s23p6 name the group this element...
Questions in other subjects:

Mathematics, 05.07.2021 19:20

English, 05.07.2021 19:20

Chemistry, 05.07.2021 19:20