
Chemistry, 26.06.2019 01:30 tylermdons
Aluminum reacts with chlorine gas to produce aluminum chloride according to the following equation. al + cl2 → alcl3 which of the following fractions can be used for the mole ratio to determine the mass of cl2 from a known mass of alcl3? a. 2/1 b. 3/2 c. 1/2 d. 2/3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Aluminum reacts with chlorine gas to produce aluminum chloride according to the following equation....
Questions in other subjects:




Biology, 11.01.2020 00:31


Mathematics, 11.01.2020 00:31




Mathematics, 11.01.2020 00:31

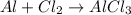
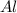 and
and 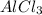 and the coefficient '3' put before the
and the coefficient '3' put before the 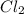 and we get the balanced chemical equation.
and we get the balanced chemical equation.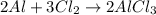
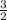 moles of
moles of 

