
Chemistry, 26.06.2019 01:30 BeenPaidGLO
What mass of fe can be produced by the reaction of 75.0 g co with excess fe2o3 according to the equation: fe2o3(s) + co(g) → fe(s) + co2(g)? a. 49.8 g fe b. 99.7 g fe c. 224 g fe d. 299 g fe

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
You know the right answer?
What mass of fe can be produced by the reaction of 75.0 g co with excess fe2o3 according to the equa...
Questions in other subjects:

History, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30



Mathematics, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30



Health, 08.01.2021 22:30

Arts, 08.01.2021 22:30

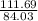 ×75.0 = 99.687g of Fe or 99.7g of Fe.
×75.0 = 99.687g of Fe or 99.7g of Fe.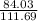 ×49.8 = 37.467g of CO should react. Thus the option is wrong.
×49.8 = 37.467g of CO should react. Thus the option is wrong.

