
Chemistry, 26.06.2019 08:00 potternatalie90
What precipitate forms when silver nitrate and potassium chromate solutions are mixed? explain. a. ag2cro4( s) b. k2cro4( s) c. agno3( s) d. kno3( s) an answer would be really appreciated! need it as soon as possible!

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
What precipitate forms when silver nitrate and potassium chromate solutions are mixed? explain. a....
Questions in other subjects:


Chemistry, 21.06.2021 03:50


Social Studies, 21.06.2021 03:50

Mathematics, 21.06.2021 03:50

English, 21.06.2021 03:50


Mathematics, 21.06.2021 03:50


Mathematics, 21.06.2021 03:50

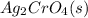
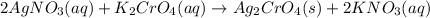
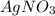 combines with potassium chromate
combines with potassium chromate 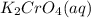 ,they undergo double displacement reaction in which exchange of ions take place.
,they undergo double displacement reaction in which exchange of ions take place.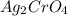 which is insoluble in water and thus formed as precipitate and potassium nitrate
which is insoluble in water and thus formed as precipitate and potassium nitrate 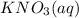 is soluble in water.
is soluble in water.

