

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Refer to the balanced equation. ( quick) 8h2s+8cl2→s8+16hcl how many l of chlorine gas, at stp, wer...
Questions in other subjects:

Mathematics, 07.05.2021 21:10


Mathematics, 07.05.2021 21:10

Arts, 07.05.2021 21:10

Spanish, 07.05.2021 21:10

Mathematics, 07.05.2021 21:10

English, 07.05.2021 21:10

Mathematics, 07.05.2021 21:10


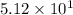 L
L 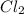
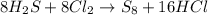
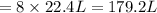 of chlorine gas.
of chlorine gas.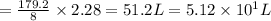 of chlorine gas.
of chlorine gas.


