
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown below. c3h7oh(l)+4.5o2(g)-> 3co2(g)+4h2o(l)deltah=-2,021kj below is a list of sentences that describe a chemical reaction. choose all of the sentences that apply to the above reaction. check all that apply. view available hint(s) check all that apply. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 4,042 kj this process is endothermic. this process is exothermic. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is -4,042 kj the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 2,021 kj this chemical reaction transfers heat from the surroundings to the system. the enthalpy for 2c3h7oh(g)+9o2(g)-> 6co2(g)+8h2o(l) is -2,021 kj this chemical reaction transfers heat from the system to the surroundings.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, jrjimenez
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
You know the right answer?
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown be...
Questions in other subjects:

Mathematics, 02.07.2019 05:00



Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00


Social Studies, 02.07.2019 05:00

History, 02.07.2019 05:00

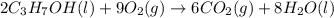 is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.
is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.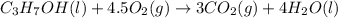
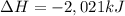
 undergoes combustion to release 2,021kJ of heat.
undergoes combustion to release 2,021kJ of heat.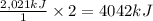 of heat.
of heat.

