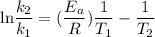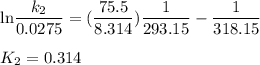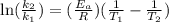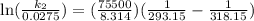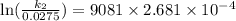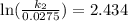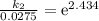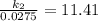Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kitt90335
Asample contains 16.75 g of the radioisotope u-236 and 50.25 g of its daughter isotope, th-232. how long did it take for decay to take place if one half-life of u-236 is 23 million years? 46 million years 69 million years 92 million years 115 million years
Answers: 3

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3


Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Acertain first-order reaction has a rate constant of 2.75 10-2 s−1 at 20.°c. what is the value of k...
Questions in other subjects:

Mathematics, 21.01.2021 17:10





Computers and Technology, 21.01.2021 17:10


Chemistry, 21.01.2021 17:10


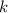 (rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.
(rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.