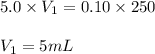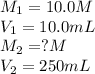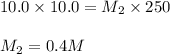Chemistry, 26.06.2019 19:00 tyneshiajones124
(a. what volume of 2.50 m lead(ii)nitrate solution contains 0.0500 mol of pb2+? ( b. how many milliliters of 5.0 m k2cr2o7 solution must be diluted to prepare 250 ml of 0.10 m solution? ( c. if 10.0 ml of a 10.0 m stock solution of naoh is diluted to 250 ml, what is the concentration of the resulting stock solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
(a. what volume of 2.50 m lead(ii)nitrate solution contains 0.0500 mol of pb2+? ( b. how many milli...
Questions in other subjects:

History, 04.01.2020 06:31


Mathematics, 04.01.2020 06:31

Biology, 04.01.2020 06:31


Mathematics, 04.01.2020 06:31

Mathematics, 04.01.2020 06:31

English, 04.01.2020 06:31

Mathematics, 04.01.2020 06:31

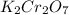 solution is 5 mL
solution is 5 mL

 ......(1)
......(1)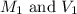 are the molarity and volume of the concentrated solution
are the molarity and volume of the concentrated solution are the molarity and volume of diluted solution
are the molarity and volume of diluted solution