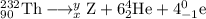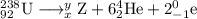Chemistry, 26.06.2019 19:30 chrissy5189
Write down the values of m (atomic number or atomic weight) and z (atomic number) for : (1) the element x formed from thorium-232 after six a and four b emissions? (2) the element y formed from uranium-238 after six a and two b emissions. (z=90 for thorium ; z=92 for uranium)

Answers: 1


Other questions on the subject: Chemistry




You know the right answer?
Write down the values of m (atomic number or atomic weight) and z (atomic number) for : (1) the ele...
Questions in other subjects:

History, 12.02.2021 06:10

Mathematics, 12.02.2021 06:10

Social Studies, 12.02.2021 06:10



History, 12.02.2021 06:10

Mathematics, 12.02.2021 06:10


Mathematics, 12.02.2021 06:10