
Chemistry, 26.06.2019 22:00 ineedtopeebeforethec
Given the following chemical reaction: 2 o2 + ch4 co2 + 2 h2o what mass of co2 is produced in the reaction of 4 moles of ch4 in excess o2? 6 c 12.01 carbon 1 h 1.01 hydrogen 8 o 16.00 oxygen a. 44 grams b. 64 grams c. 164 grams d. 176 grams

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 08:50, carlinryan
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2

Chemistry, 23.06.2019 18:10, rafrod75
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2

Chemistry, 23.06.2019 18:30, cyniakaofficial
Describe two techniques used to measure the ph of a solution
Answers: 2
You know the right answer?
Given the following chemical reaction: 2 o2 + ch4 co2 + 2 h2o what mass of co2 is produced in the r...
Questions in other subjects:

English, 25.04.2020 21:21

Mathematics, 25.04.2020 21:21



History, 25.04.2020 21:21

History, 25.04.2020 21:21

English, 25.04.2020 21:21

Social Studies, 25.04.2020 21:21


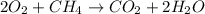
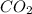
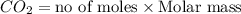
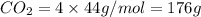
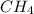 in excess
in excess 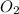 .
.

