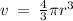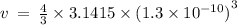Chemistry, 27.06.2019 02:00 msbanks317
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calculate the fraction of space that xe atoms occupy in a sample of xenon at stp.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calcula...
Questions in other subjects:

Mathematics, 18.05.2021 14:50


Chemistry, 18.05.2021 14:50

Mathematics, 18.05.2021 14:50



Mathematics, 18.05.2021 14:50