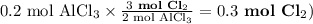Chemistry, 27.06.2019 04:00 tracyaleblanc
*explain your answer for brainliestaluminum reacts with chlorine gas to produce aluminum chloride according to the following equation. al + cl2 → alcl3which of the following fractions can be used for the mole ratio to determine the mass of cl2 from a known mass of alcl3? two over one three over two one over two two over three

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
You know the right answer?
*explain your answer for brainliestaluminum reacts with chlorine gas to produce aluminum chloride ac...
Questions in other subjects:


Mathematics, 02.12.2020 17:50

History, 02.12.2020 17:50

Biology, 02.12.2020 17:50


Mathematics, 02.12.2020 17:50


Chemistry, 02.12.2020 17:50

Mathematics, 02.12.2020 17:50

History, 02.12.2020 17:50