
Chemistry, 27.06.2019 04:30 saskiat1155
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-], and hydronium, [h3o+], ions. b) pure water contains larger amounts of hydroxide, [oh-], ions than hydronium, [h3o+], ions. c) pure water contains larger amounts of hydronium, [h3o+], ions than hydroxide, [oh-], ions. d) pure water is an electrolyte.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2


Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 01:30, josephaciaful
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1
You know the right answer?
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-]...
Questions in other subjects:

Mathematics, 28.01.2021 01:00

English, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00

English, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

History, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

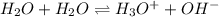
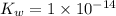
![[H_3O^+]=[OH^-]](/tpl/images/0022/0619/f28a0.png)
![K_w=1\times 10^{-14}=[H_3O^+]\times [OH^-]](/tpl/images/0022/0619/a50f9.png)
![[H_3O^+]=10^{-7}](/tpl/images/0022/0619/e211a.png)
![pH=-\log[H_3O^+]=-\log[10^{-7}] = 7](/tpl/images/0022/0619/ddc2d.png)


