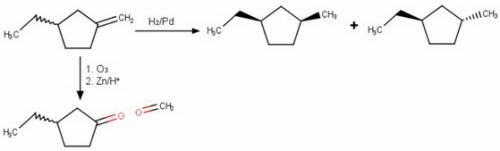
Compound x has the formula c8h14. x reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a mixture of cis- and trans-1-ethyl-3-methylcyclopentane. treatment of x with ozone followed by zinc in aqueous acid gives a ketone and formaldehyde (ch2=o). what is the structure of x?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2



You know the right answer?
Compound x has the formula c8h14. x reacts with one molar equivalent of hydrogen in the presence of...
Questions in other subjects:





Mathematics, 01.09.2019 07:30

Mathematics, 01.09.2019 07:30

Mathematics, 01.09.2019 07:30

Physics, 01.09.2019 07:30


Health, 01.09.2019 07:30