
Chemistry, 27.06.2019 19:30 ccarwile01
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. (4 points) a + b yieldsproducts trial [a] [b] rate 1 0.10 m 0.20 m 1.2 × 10-2 m/min 2 0.10 m 0.40 m 4.8 × 10-2 m/min 3 0.20 m 0.40 m 9.6 × 10-2 m/min

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions in other subjects:


Mathematics, 20.12.2020 06:40


Mathematics, 20.12.2020 06:40




Mathematics, 20.12.2020 06:40


English, 20.12.2020 06:40

![k[A]^1[B]^2](/tpl/images/0024/4376/f6c70.png) , order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is
, order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is 
![Rate=k[A]^x[B]^y](/tpl/images/0024/4376/ddde1.png)
![1.2\times 10^{-2}=k[0.10]^x[0.20]^y](/tpl/images/0024/4376/c938a.png) (1)
(1)![4.8\times 10^{-2}=k[0.10]^x[0.40]^y](/tpl/images/0024/4376/a7ba9.png) (2)
(2)![\frac{4.8\times 10^{-2}}{1.2\times 10^{-2}}=\frac{k[0.10]^x[0.40]^y}{k[0.10]^x[0.20]^y}](/tpl/images/0024/4376/b4c1f.png)
 therefore y=2.
therefore y=2.![9.6\times 10^{-2}=k[0.20]^x[0.40]^y](/tpl/images/0024/4376/18d47.png) (4)
(4)![\frac{9.6\times 10^{-2}}{4.8\times 10^{-2}}=\frac{k[0.20]^x[0.40]^y}{k[0.10]^x[0.40]^y}](/tpl/images/0024/4376/61313.png)
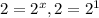 , x=1
, x=1![Rate=k[A]^1[B]^2](/tpl/images/0024/4376/ca297.png)
![1.2\times 10^{-2}=k[0.10]^1[0.20]^2](/tpl/images/0024/4376/f6a0d.png)
 .
.

