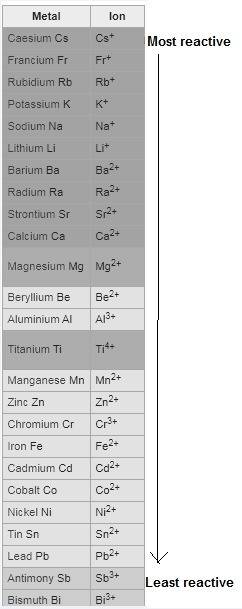
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can replace aluminum (al) in the compound because lead is lower on the activity series. true false question 2(multiple choice worth 4 points) (04.03 mc) which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh? na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced question 3(multiple choice worth 4 points) (04.03 lc) which of the following is a single replacement reaction? ba(oh)2 + h2so4 → baso4 + 2h2o 2mg + o2 → 2mgo h2o+ co2 → h2co3 zn + h2so4 → znso4 + h2 question 4(multiple choice worth 4 points) (04.03 mc) sodium metal reacts with water to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is more reactive than hydrogen. a single replacement reaction takes place because sodium is more reactive than hydrogen. question 5 (true/false worth 2 points) (04.03 lc) a single replacement reaction is a reaction in which one element replaces a similar element within a compound. true false question 6(multiple choice worth 4 points) (04.03 mc) the table shows the nature of reactants and products formed in a certain type of chemical reaction. nature of reactants and products reactants products metal + ionic compound metal + ionic compound which of the following is true about the type of chemical reaction? it is a single replacement reaction, and the anions in the two ionic compounds are different. it is a single replacement reaction, and the cations in the two ionic compounds are different. it is a double replacement reaction, and the anions in the two ionic compounds are different. it is a double replacement reaction, and the cations in the two ionic compounds are different.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 07:00, ultimatesaiyan
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1

Chemistry, 23.06.2019 10:00, lexusdixon3
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1

Chemistry, 23.06.2019 14:00, RichardKing2376
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
You know the right answer?
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can re...
Questions in other subjects:





Computers and Technology, 09.10.2019 20:00

Computers and Technology, 09.10.2019 20:00




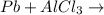 no reaction
no reaction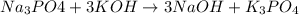 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction. 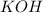 as well as
as well as 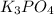
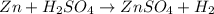
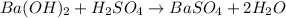 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place. 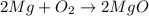 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.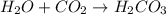 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.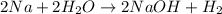 Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen. 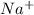 in NaOH and
in NaOH and 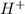 get reduced to give
get reduced to give 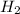
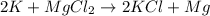 where K is a metal and
where K is a metal and 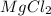 is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.
is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.