
Chemistry, 28.06.2019 02:30 Austin4094
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and water. what best describes this reaction? a single replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a single replacement reaction occurs because potassium is more reactive than hydrogen. a double replacement reaction occurs because potassium is less reactive than hydrogen.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 06:30, nikeahbrown
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and...
Questions in other subjects:

Chemistry, 27.01.2020 03:31



Mathematics, 27.01.2020 03:31


Mathematics, 27.01.2020 03:31



Mathematics, 27.01.2020 03:31

Chemistry, 27.01.2020 03:31


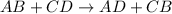
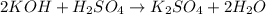
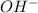 ion of KOH combines with
ion of KOH combines with 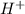 ion of sulfuric acid to give water.
ion of sulfuric acid to give water.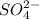 ) of sulfuric acid gets combine with two potassium ion (
) of sulfuric acid gets combine with two potassium ion (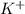 )to give salt of potassium sulfate,
)to give salt of potassium sulfate, 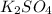 .
.

