Chemistry, 28.06.2019 03:30 yhbgvfcd6677
3. given the following reactants: agi + fe(co3)3 • predict the products • balance the equation • state what type of reaction this is.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, chloe081
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p. balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2



Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1
You know the right answer?
3. given the following reactants: agi + fe(co3)3 • predict the products • balance the equation...
Questions in other subjects:


Computers and Technology, 11.01.2020 04:31


Mathematics, 11.01.2020 04:31

History, 11.01.2020 04:31


Biology, 11.01.2020 04:31


Mathematics, 11.01.2020 04:31

Mathematics, 11.01.2020 04:31

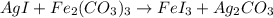
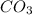 = 3
= 3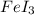 by 2 and
by 2 and 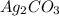 by 3 on the product side. Therefore, balanced chemical equation will be as follows.
by 3 on the product side. Therefore, balanced chemical equation will be as follows.