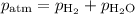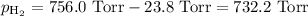Chemistry, 28.06.2019 07:30 emmaty7845
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr and the temperature is 25 °c. the vapor pressure of water at various temperatures can be found in this table. calculate the molar mass of the metal.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2


Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291...
Questions in other subjects:




Mathematics, 12.07.2019 22:30






Social Studies, 12.07.2019 22:30