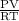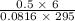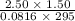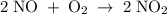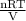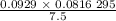Chemistry, 28.06.2019 07:30 nickeymcorrea
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.500 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 °c. what are the partial gasses of no, and no2?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l,...
Questions in other subjects:

Mathematics, 10.11.2020 14:40



English, 10.11.2020 14:40

Mathematics, 10.11.2020 14:40


English, 10.11.2020 14:40


Mathematics, 10.11.2020 14:50

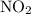 is 0.400 atm at the end of the reaction.
is 0.400 atm at the end of the reaction.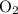 initially are;
initially are;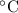 = 273 + 22 K = 295 K
= 273 + 22 K = 295 K