
Chemistry, 28.06.2019 17:30 Itsyourgirllulu
You wish to measure the iron content of the well water on the new property you are about to buy. you prepare a reference standard fe3 solution with a concentration of 5.17 ă— 10-4 m. you treat 13.0 ml of this reference with hno3 and excess kscn to form a red complex, and dilute the reference to 45.0 ml. the diluted reference is placed in a cell with a 1.00-cm light path. you then take 30.0 ml of the well water, treat with hno3 and excess kscn, and dilute to 100.0 ml. this diluted sample is placed in a variable pathlength cell. the absorbance of the reference and the sample solutions match when the pathlength is 4.78 cm. what is the concentration of iron in the well water? for each solution, the zero is set with a blank.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
You know the right answer?
You wish to measure the iron content of the well water on the new property you are about to buy. you...
Questions in other subjects:

Mathematics, 07.05.2020 08:02

English, 07.05.2020 08:02

Chemistry, 07.05.2020 08:02




History, 07.05.2020 08:02

History, 07.05.2020 08:02

Mathematics, 07.05.2020 08:02

 M
M by the Beer-Lambert law, where
by the Beer-Lambert law, where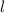 the path length, ε the molar absorptivity of the solute, and
the path length, ε the molar absorptivity of the solute, and concentration of the solution.
concentration of the solution. is constant;
is constant; 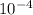 M. Diluting it to 45.0 mL results in a concentration of
M. Diluting it to 45.0 mL results in a concentration of 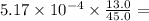 1.494 M.
1.494 M.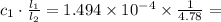 3.126 M.
3.126 M. 1.04 ⨯
1.04 ⨯ 

