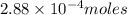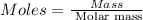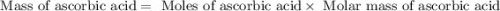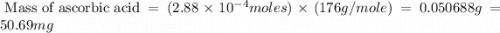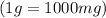Chemistry, 28.06.2019 17:30 kprincess16r
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.88×10−4 mol of ascorbic acid. to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.88×10−4 mol of ascorbic acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, princessroyal
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions in other subjects:


Physics, 26.01.2022 02:40

Mathematics, 26.01.2022 02:40


English, 26.01.2022 02:40


Mathematics, 26.01.2022 02:40