
Chemistry, 28.06.2019 18:00 Lacrosse34
The hydrides of group 5a are nh3, ph3, ash3, and sbh3. arrange them from highest to lowest boiling point. rank the molecules from highest to lowest boiling point. to rank items as equivalent, overlap them.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 11:00, jdisalle2808
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
The hydrides of group 5a are nh3, ph3, ash3, and sbh3. arrange them from highest to lowest boiling p...
Questions in other subjects:


Mathematics, 09.07.2019 17:10



History, 09.07.2019 17:10

Mathematics, 09.07.2019 17:10

Mathematics, 09.07.2019 17:10



Geography, 09.07.2019 17:10

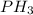 to
to 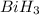 . But boiling point of
. But boiling point of 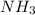 is higher than rest of the hydrides of group 5A.
is higher than rest of the hydrides of group 5A. 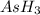 but lower than
but lower than 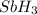 and
and 

