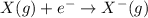Chemistry, 28.06.2019 18:30 nayelimoormann
Elaborate on how electron affinity and ion formation relate. a) electron affinity is the energy required to add one electron to an atom forming an anion. b) electron affinity is the energy required to remove one electron from an atom forming a cation. c) electron affinity is the energy produced when adding an electron to an atom forming an isotope. d) electron affinity is the energy donated to discharge one electron from an atom forming a baryon.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

You know the right answer?
Elaborate on how electron affinity and ion formation relate. a) electron affinity is the energy requ...
Questions in other subjects:

Health, 08.06.2021 14:00



Social Studies, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

English, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

Engineering, 08.06.2021 14:00

English, 08.06.2021 14:00