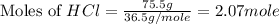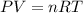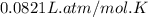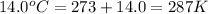Chemistry, 28.06.2019 19:30 AnastasiaJauregui
What is the mass of solid nh4cl formed when 75.5g of nh3 is mixed with an equal mass of hcl? what is the volume of the gas remaining, measured at 14.0c and 752 mmhg? what gas is it? the formula is nh3 (g) + hcl gas -> nh4cl solid

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
What is the mass of solid nh4cl formed when 75.5g of nh3 is mixed with an equal mass of hcl? what i...
Questions in other subjects:



Mathematics, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00

Biology, 02.03.2021 21:00

Biology, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00

Social Studies, 02.03.2021 21:00


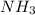 and HCl.
and HCl.
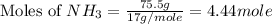

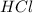 = 36.5 g/mole
= 36.5 g/mole