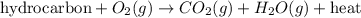Chemistry, 28.06.2019 22:00 aletadaboss
What is not true about most combustion reactions? a) elemental carbon is a product b) energy is released c) a carbon-based fuel is a reactant d) molecular oxygen is a reactant

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2


Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
What is not true about most combustion reactions? a) elemental carbon is a product b) energy is rel...
Questions in other subjects:


Geography, 23.06.2019 05:00


Biology, 23.06.2019 05:00


Physics, 23.06.2019 05:00