
Chemistry, 29.06.2019 02:30 joshuakirby
Calculate how many grams of sodium azide (nan3) are needed to inflate a 25.0 × 25.0 × 20.0 cm bag to a pressure of 1.35 atm at a temperature of 20.0°c. how much sodium azide is needed if the air bag must produce the same pressure at 10.0°c? the equation is 20nan3+6sio2+4kno3=32n2+5na4sio4+k4 sio

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Calculate how many grams of sodium azide (nan3) are needed to inflate a 25.0 × 25.0 × 20.0 cm bag to...
Questions in other subjects:



English, 23.04.2020 19:49

Mathematics, 23.04.2020 19:50



History, 23.04.2020 19:50



Health, 23.04.2020 19:50

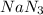 at temperature
at temperature  28.47 g.
28.47 g. 29.51 g.
29.51 g.
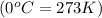


 .
.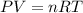



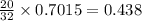 moles of
moles of 
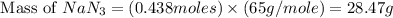
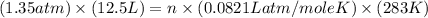

 moles of
moles of 


