
Chemistry, 29.06.2019 08:30 Calumworthy6046
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k and total pressure 1.00 bar. calculate the amounts of the components in the mixture at equilibrium given that k = 870 for the reaction h2 (g) + i2 (g) ⇜ 2 hi (g).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k an...
Questions in other subjects:


Mathematics, 10.06.2021 06:30



Biology, 10.06.2021 06:30





![[HI] _{eq}=0.825mol](/tpl/images/0030/3484/a93b2.png)
![[H_2] _{eq}=0.010mol](/tpl/images/0030/3484/29662.png)
![[I_2] _{eq}=0.078mol](/tpl/images/0030/3484/3a542.png)
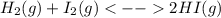
 ):
):![\frac{Kp}{(RT)^{2-2} }=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}} \\Kp=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}}](/tpl/images/0030/3484/98372.png)
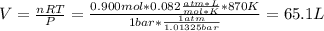
![[H_2] _0=\frac{0.300mol}{65.1L}=0.005M](/tpl/images/0030/3484/0ef57.png)
![[I_2] _0=\frac{0.400mol}{65.1L}=0.006M](/tpl/images/0030/3484/d9a8e.png)
![[HI] _0=\frac{0.200mol}{65.1L}=0.003M](/tpl/images/0030/3484/e3fd2.png)
 due to the equilibrium:
due to the equilibrium: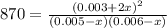

![[HI] _{eq} =(0.003M +2(0.00484M))*65.1L=0.825mol](/tpl/images/0030/3484/1d681.png)
![[H_2] _{eq} =(0.005M -0.00484M)*65.1L=0.010mol](/tpl/images/0030/3484/34688.png)
![[I_2] _{eq} =(0.006M -0.00484M)*65.1L=0.078mol](/tpl/images/0030/3484/28b57.png)



